APYX Medical Corporation, the manufacturer of a helium and radiofrequency plasma technology marketed and sold as Renuvion, has received the authorization of 510 (K) of the United States Food and Medicines Administration for the Body Body of Ayon’s contouring system. The company prepares for a commercial launch of Ayon with key surgeons in critical geographies from the second half of 2025.
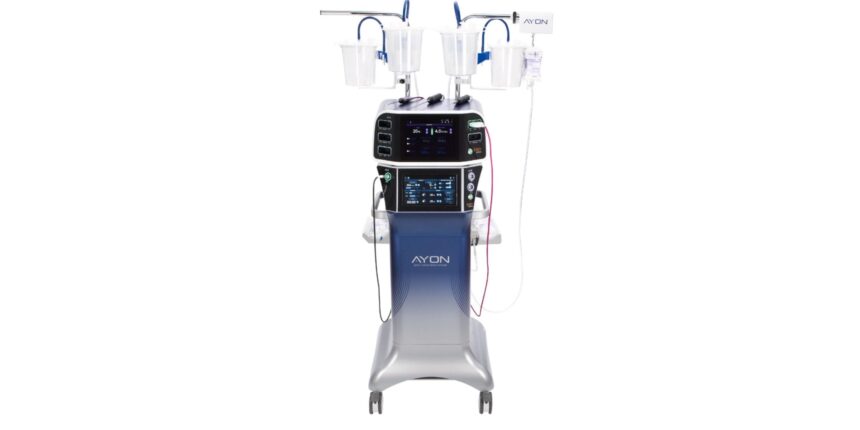
Ayon is a body contour system designed by the surgeon that integrates the elimination of fats, the closed circuit contour, the contraction of the tissues and the electro -surgical capabilities. The system includes characteristics such as lifting technology for real -time adjustments and renuvion for improved tissue contraction.
This initial authorization 510 (K) for Ayon covers a variety of aesthetic treatments, including renavion to address loose and lax skin, ultrasound -assisted liposuction, electroculation to support excess tissue. The company plans to expand the authorized indications for Ayon to include power liposuction, with an additional presentation of 510 (k) at the end of this year.
“Receive the authorization of the FDA for the contour system of Ayon’s body, which includes our owner Renuvion technology for the treatment of loose skin and lax, marks a big step forward in our mission of training surgeons interforcement Power tool in a launch.
Goodwin points out that the aesthetics market anticipates an increase in body contour procedures next year, since more than 15 million drug users GLP-1 experience rapid weight loss and look for solutions for loose skin and rescue their bodies.
“Ayon provides surgeons for all the necessary solutions on a device to meet the needs of these patients,” says Goodwin in a statement.
Photo legend: Ayon’s body contour system
Photo credit: APYX Medical Corporation